Formula For Iron 2 Oxide
elan
Sep 17, 2025 · 7 min read
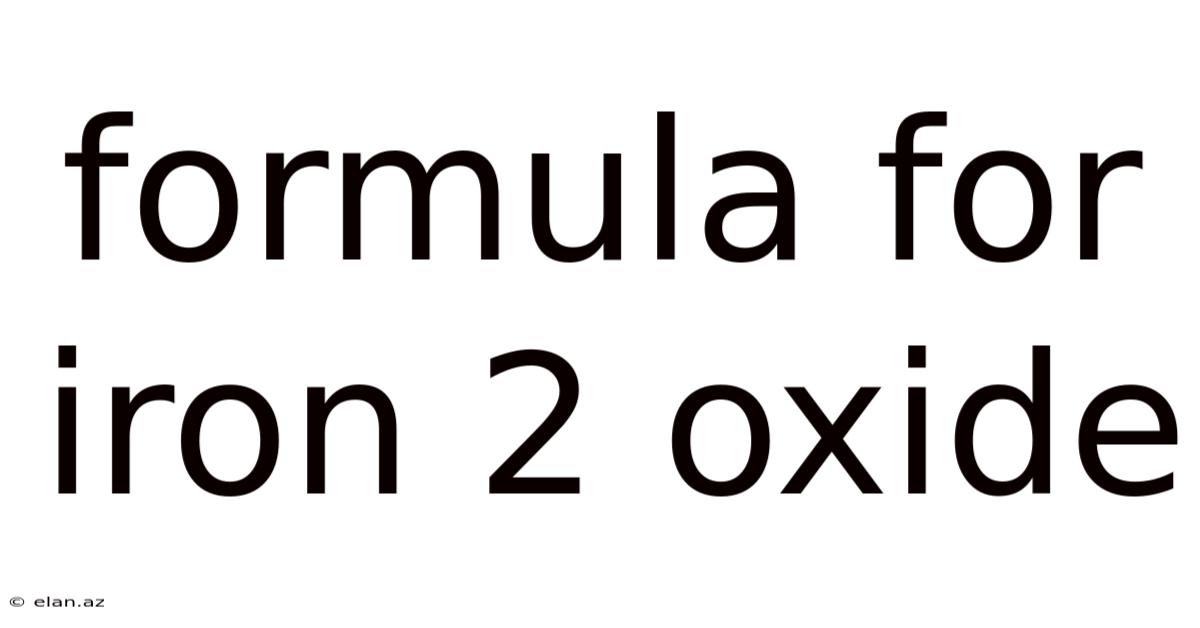
Table of Contents
Unveiling the Formula for Iron(II) Oxide: A Deep Dive into its Properties and Applications
Iron(II) oxide, also known as ferrous oxide, is a fascinating compound with a rich history and a wide range of applications. Understanding its chemical formula, properties, and uses is crucial for various fields, from materials science and chemistry to environmental studies and industrial processes. This article provides a comprehensive exploration of iron(II) oxide, delving into its formula, structure, preparation, properties, and applications, aiming to offer a clear and complete understanding for both students and professionals.
Introduction to Iron(II) Oxide
The chemical formula for iron(II) oxide is FeO. This simple formula represents a compound where one iron(II) ion (Fe²⁺) is bonded to one oxide ion (O²⁻). The Roman numeral II in parenthesis indicates the oxidation state of iron, which is +2 in this case. This contrasts with iron(III) oxide (Fe₂O₃), where iron has an oxidation state of +3. The difference in oxidation states significantly impacts the properties and applications of these two iron oxides. This article will focus specifically on the characteristics and significance of FeO.
Understanding the Chemical Formula: FeO
The formula FeO signifies a 1:1 stoichiometric ratio of iron and oxygen atoms. This means that for every one iron atom present, there is one oxygen atom. This simple ratio is a key to understanding the compound's crystal structure and properties. The formation of FeO involves the transfer of two electrons from an iron atom to an oxygen atom, resulting in the formation of ionic bonds. This ionic bonding is responsible for many of the properties of iron(II) oxide. It's important to note that perfectly stoichiometric FeO is difficult to obtain in practice due to its tendency towards non-stoichiometry. We’ll explore this further in the section discussing its properties.
Crystal Structure and Bonding
Iron(II) oxide adopts a rock salt crystal structure. This means it has a cubic close-packed arrangement of oxygen ions (O²⁻), with iron(II) ions (Fe²⁺) occupying the octahedral holes within the lattice. This structure is highly ordered and contributes to several of FeO's physical properties. The ionic bonding between Fe²⁺ and O²⁻ ions is the primary force holding the crystal lattice together. However, the nature of this bonding is somewhat complex and can be influenced by factors such as temperature and the presence of defects within the crystal structure. The relatively strong ionic bonds contribute to the high melting point of iron(II) oxide.
Preparation and Synthesis of FeO
Preparing pure iron(II) oxide can be challenging due to its tendency to oxidize to iron(III) oxide (Fe₂O₃). Several methods can be employed, but careful control of reaction conditions is crucial. Some common methods include:
-
Reduction of Iron(III) Oxide: Fe₂O₃ can be reduced by heating it in a controlled atmosphere of hydrogen gas (H₂) at high temperatures. The reaction can be represented as: Fe₂O₃ + H₂ → 2FeO + H₂O. The precise temperature and pressure must be carefully managed to prevent the formation of metallic iron.
-
Controlled Oxidation of Iron: Iron metal can be oxidized in a controlled environment with a limited supply of oxygen. This process requires precise control of temperature and oxygen partial pressure to prevent the formation of Fe₃O₄ (magnetite) or Fe₂O₃.
-
Decomposition of Iron(II) Oxalate: Heating iron(II) oxalate (FeC₂O₄) in an inert atmosphere will decompose it, producing iron(II) oxide: FeC₂O₄ → FeO + CO + CO₂. This method provides a relatively pure sample of FeO, provided the inert atmosphere is carefully maintained.
-
Solid-State Reaction: Iron(II) oxide can be synthesized through solid-state reactions, often involving the careful mixing and heating of iron(II) salts and other metal oxides under carefully controlled conditions.
The choice of method depends on factors such as desired purity, scale of production, and available resources. Each method requires meticulous control of parameters to achieve the desired product.
Properties of Iron(II) Oxide
Iron(II) oxide exhibits several key properties:
-
Appearance: Pure FeO is a black, crystalline solid. However, samples often appear dark grey or greenish due to the presence of impurities or non-stoichiometry.
-
Melting Point: FeO has a relatively high melting point, approximately 1377°C (2511°F). This high melting point is due to the strong ionic bonds within its crystal structure.
-
Non-Stoichiometry: A significant challenge in working with iron(II) oxide is its tendency toward non-stoichiometry. This means that the actual composition often deviates from the ideal 1:1 ratio of iron to oxygen. This is because some iron(II) ions can be replaced by iron(III) ions, leading to a range of compositions often represented as Fe₁₋ₓO, where x represents the deviation from stoichiometry. This deviation significantly impacts the physical and chemical properties of the material.
-
Magnetic Properties: While not as strongly magnetic as magnetite (Fe₃O₄), FeO does exhibit antiferromagnetic properties below its Néel temperature (around 198 K or -75°C). Above this temperature, it becomes paramagnetic.
-
Solubility: FeO is largely insoluble in water but reacts with acids to form iron(II) salts.
-
Reactivity: Iron(II) oxide is relatively reactive, readily oxidizing to Fe₃O₄ or Fe₂O₃ in the presence of oxygen. This susceptibility to oxidation is a significant factor in its applications and handling.
Applications of Iron(II) Oxide
The applications of iron(II) oxide are diverse, stemming from its unique properties and reactivity. Some key applications include:
-
Ceramic Industry: FeO is used as a pigment in ceramics and glass, contributing to the coloration of these materials. It can produce a variety of colors depending on the oxidizing conditions during firing.
-
Catalyst: Iron(II) oxide acts as a catalyst in various chemical reactions, particularly in processes involving oxidation or reduction.
-
Magnetic Materials: While not as widely used as other iron oxides in magnetic applications, FeO's magnetic properties have niche applications in specific magnetic materials.
-
Steelmaking: Although not directly added, FeO plays an indirect role in steelmaking through its presence in iron ores and its influence on the reduction process.
-
Geochemistry and Mineralogy: FeO is a crucial component in many geological formations and minerals. Understanding its behavior is essential for geochemical modeling and mineral exploration.
-
Powder Metallurgy: FeO finds applications in powder metallurgy, contributing to the properties of sintered components.
-
Synthesis of other Iron Compounds: FeO serves as a precursor for the synthesis of other iron compounds.
The specific application often depends on the purity and stoichiometry of the FeO sample, highlighting the importance of precise control during preparation and handling.
Frequently Asked Questions (FAQ)
-
What is the difference between Iron(II) oxide and Iron(III) oxide? The key difference lies in the oxidation state of iron. Iron(II) oxide (FeO) contains iron in the +2 oxidation state, while Iron(III) oxide (Fe₂O₃) contains iron in the +3 oxidation state. This difference leads to significant variations in their properties and applications.
-
Is FeO toxic? While not acutely toxic in small quantities, prolonged exposure to iron(II) oxide dust can cause respiratory irritation. Appropriate safety measures should be taken when handling it.
-
Why is it difficult to obtain pure FeO? The difficulty stems from its tendency towards non-stoichiometry and its ease of oxidation to higher iron oxides (Fe₃O₄ and Fe₂O₃). Controlling the atmosphere and temperature during preparation is crucial for obtaining a sample with the desired composition.
-
What are the environmental implications of FeO? The environmental impact of FeO is generally considered low. However, its release into the environment can contribute to soil and water contamination if not properly managed.
-
What are the future prospects for FeO research and applications? Ongoing research focuses on improving methods for producing high-purity FeO, understanding its non-stoichiometric behavior, and exploring novel applications in areas such as catalysis and energy materials.
Conclusion
Iron(II) oxide (FeO), despite its seemingly simple formula, is a complex and fascinating compound with a broad range of applications. Understanding its crystal structure, properties, preparation methods, and potential for non-stoichiometry is crucial for utilizing its potential across various fields. While challenges remain in obtaining perfectly stoichiometric FeO, ongoing research continues to uncover its potential and refine its applications, ensuring its continued significance in materials science, chemistry, and beyond. This article has hopefully provided a comprehensive overview, equipping readers with a deeper understanding of this important compound and its implications. Further exploration into specific applications or related research can provide even more detailed insights into this multifaceted material.
Latest Posts
Latest Posts
-
Predict Crossword Clue 8 Letters
Sep 17, 2025
-
Calculate Bond Energy From Enthalpy
Sep 17, 2025
-
Gallons In A Cubic Foot
Sep 17, 2025
-
Air Force Salary For Officers
Sep 17, 2025
-
Relative Mass Of Subatomic Particles
Sep 17, 2025
Related Post
Thank you for visiting our website which covers about Formula For Iron 2 Oxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.